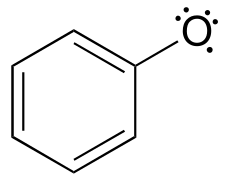
16: Free radical reactions
Contents
16: Free radical reactions#

photo credit: https://www.flickr.com/photos/stevehopson/
Introduction
Imagine that you are an 18th century British sailor about set out with Commodore George Anson to raid Spanish shipping fleets in the Pacific. You know full well that you are signing up for a long and arduous ordeal, with months of constant seasickness, bad food, cramped, unsanitary conditions, and brutal warfare. You are mentally ready for these hardships, but what you are not prepared for is to watch your own body rot away – to literally fall apart.
Below is a description of the suffering endured by many sailors of the time:
Some lost their very substance and their legs became swollen and puffed up while the sinews contracted and turned coal-black and, in some cases, all blotched with drops of purplish blood. Then the disease would creep up to the hips, thighs and shoulders, arms and neck. And all the sick had their mouths so tainted and their gums so decayed that the flesh peeled off down to the roots of their teeth, which almost all fell out…
There were devastating neurological as well as physiological effects. Scurvy had the ability to inhibit a person’s normal restraints on emotion: they became intensely homesick and nostalgic, wept at the slightest disappointment, and screamed in agony upon smelling the scent of flower blossoms drifting across the water from a nearby shore.
The disease afflicting the sailors was scurvy, which we now know is caused by a deficiency of vitamin C in the diet. European sea voyagers in the 18th century and earlier subsisted mainly on a diet of salted meat, hard biscuits, pea soup, oatmeal, and beer. After the first couple of weeks at sea, fresh fruits and vegetables - and the nutrients they contained – were all consumed or spoiled. The salted meat and hardtack diet provided salt and calories, but little else of nutritional value.
Although it is rare now, scurvy has plagued sailors for centuries, with records of its occurrence on ships going back as far as the 15th century voyages of Magellan and Vasco de Gama, both of whom lost up to three quarters of their crew to the disease on long ocean crossings. Various cultures made the connection between scurvy and diet, and learned effective preventative measures: sailors with the 16th century French explorer Jacques Cartier, for example, were cured of their scurvy upon arriving in Canada and taking the advice of native people to eat the leaves and bark of pine trees. These were lessons, unfortunately, that often had to be relearned time and again, as the knowledge gained by one culture was not effectively recorded and passed along to others.
Vitamin C, or ascorbic acid as it is known to chemists, plays an essential helping role in a variety of essential biochemical reactions. Most living things are able to synthesize ascorbic acid – the exceptions include humans and other higher primates, several species of bats, and some rodents such as guinea pigs and capybaras. Humans lack the last enzyme in the ascorbic acid biosynthetic pathway, L-gulonolactone oxidase. (EC 1.1.3.8) (You were invited to propose the mechanism for this redox reaction in problem 15.10).
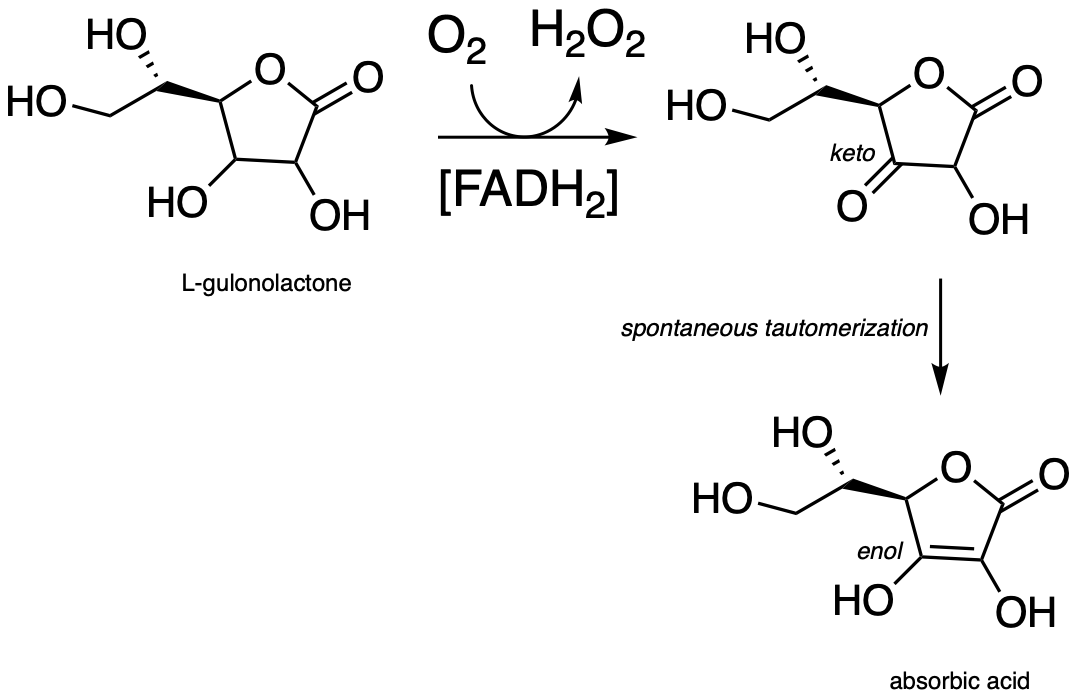
fig 1a
Because we cannot make our own ascorbic acid, we need to get it in our diet. It is abundant in many plant-based foods, citrus fruits in particular. The traditional diet of the Inuit people of the arctic region contains virtually no plant products, but vitamin C is obtained from foods such as kelp, caribou livers, and whale skin. For a time in the 18th century, the observation that citrus fruits quickly cured scurvy led to the practice of including in a ship’s stores a paste prepared from boiled lemon juice. Unfortunately, ascorbic acid did not survive the boiling process, rendering the paste ineffective against scurvy. Captain James Cook, the legendary explorer and the first European to make it to the east coast of Australia and the Hawaiian islands, brought along sourkraut (fermented cabbage), a somewhat more effective vitamin C supplement. According to his own account, Cook’s sailors at first refused to eat the pungent preparation, so the captain engaged in a little psychological trickery: he declared that it would only be served to officers. The enlisted sailors quickly took offense, and demanded their own sourkraut ration. Later, the British navy famously adopted the practice of adding lemon or lime juice to their ships’ rum rations, leading to the birth of the slang term ‘Limey’ used by European and American sailors to refer to their British counterparts.
***
The biochemical role of ascorbic acid is to facilitate the transfer of single electrons in a variety of redox reactions - note here the emphasis on single electrons, as opposed to the redox reactions we studied in chapter 15 in which electrons were transferred in pairs. The subject of this chapter is single-electron chemistry, and the free radical intermediates that are involved in single electron reaction steps.
Later in this chapter we will learn the chemical details of why ascorbic acid deficiency causes scurvy, how the act of breathing makes you get old, how polystyrene packing foam is made, and other interesting applications of single-electron chemistry. But first we need to cover some basics ideas about single electron chemical steps, and the free radical intermediates that result from them.
Section 16.1: Overview of single-electron reactions and free radicals#
Beginning with acid-base reactions in chapter x and continuing though the chapters on nucleophilic substitution, carbonyl addition, acyl substitution, α-carbon chemistry, and electrophilic reactions , we have been studying reaction mechanisms in which both electrons in a covalent bond or lone pair move in the same direction. In this chapter, we learn about reactions in which the key steps involve the movement of single electrons. Single electron movement is depicted by single-barbed ‘fish-hook’ arrows (as opposed to the familiar double-barbed arrows that we have been using throughout the book to show two-electron movement).
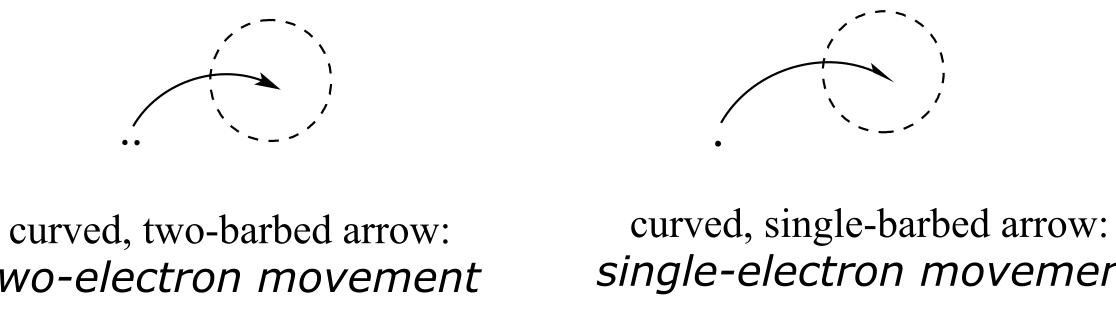
fig 1
Single-electron mechanisms involve the formation and subsequent reaction of free radical species, highly unstable intermediates that contain an unpaired electron. Free radicals are often formed from homolytic cleavage, an event in which the two electrons in a breaking covalent bond move in opposite directions. The bond in molecular chlorine, for example, is subject to homolytic cleavage when chlorine is subjected to heat or light. The result is two chlorine radicals. Note that each radical has a formal charge of zero.
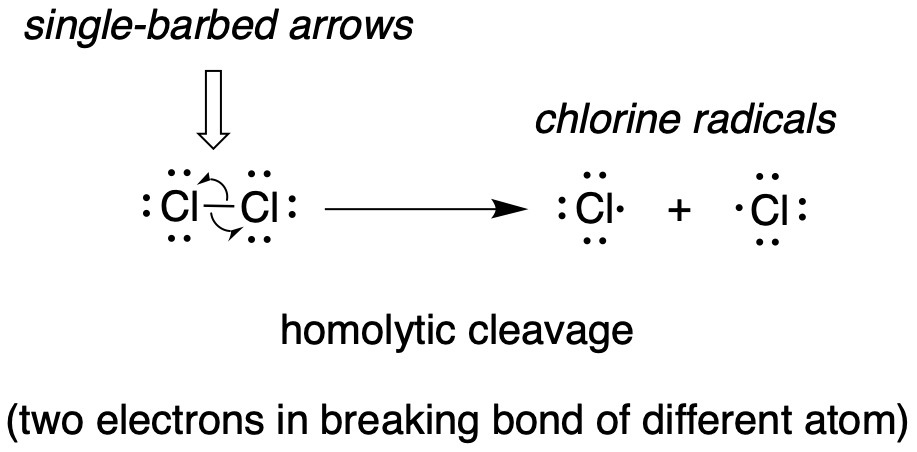
fig 2
In contrast, essentially all of the reactions we have studied up to now involve bond-breaking events in which both electrons move in the same direction: this is called heterolytic cleavage.
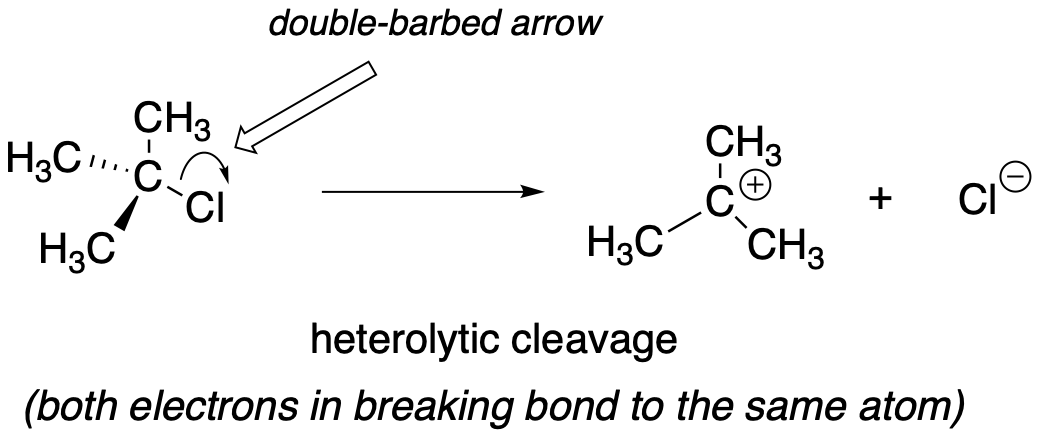
fig 3
Two other homolytic cleavage reactions that we will see in this chapter can be described as ‘radical hydrogen atom abstraction’ and ‘radical alkene addition’:
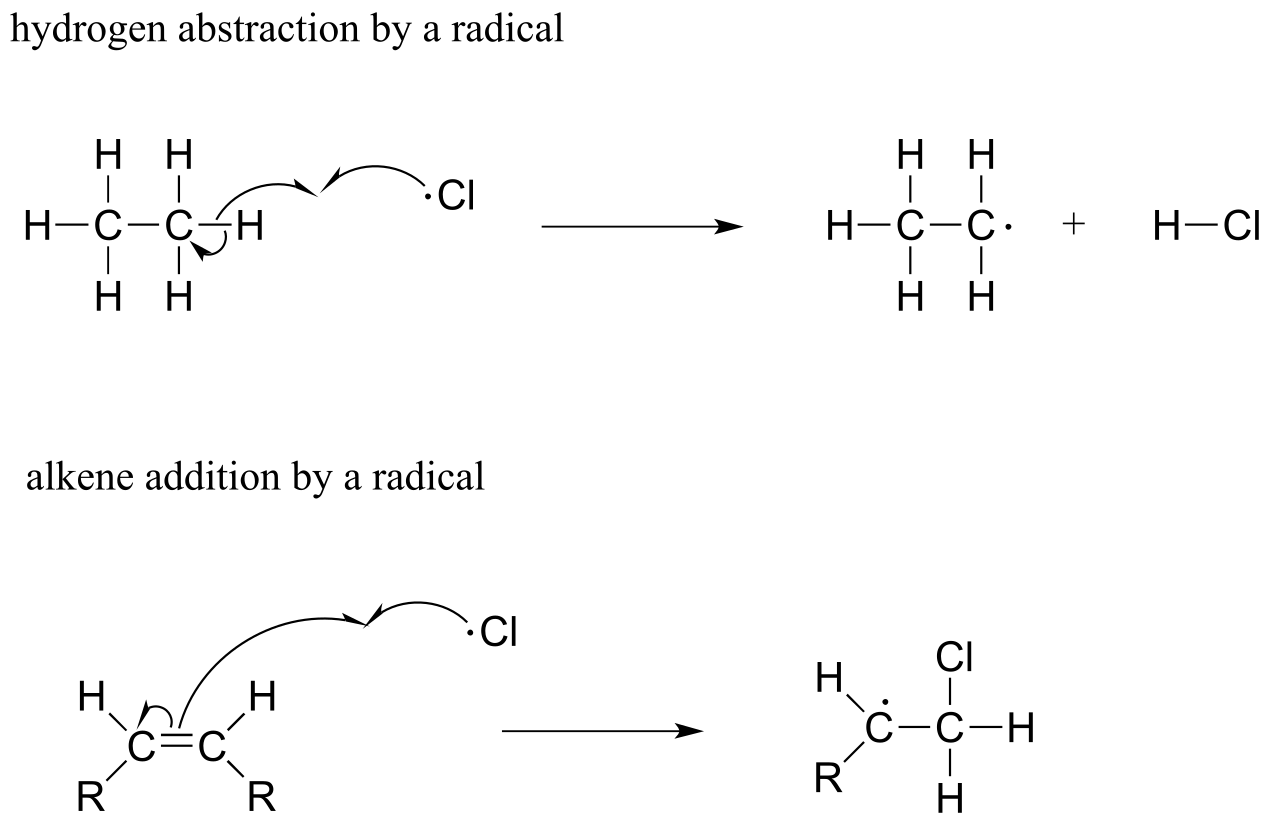
fig 4
Single-electron reaction mechanisms involve the formation of radical species, and in organic reactions these are often carbon radicals. A carbon radical is sp2 hybridized, with three σ bonds arranged in trigonal planar geometry and the single unpaired electron occupying an unhybridized p orbital. Contrast this picture with a carbocation reactive intermediate, which is also sp2 hybridized with trigonal planar geometry but with an empty p orbital.
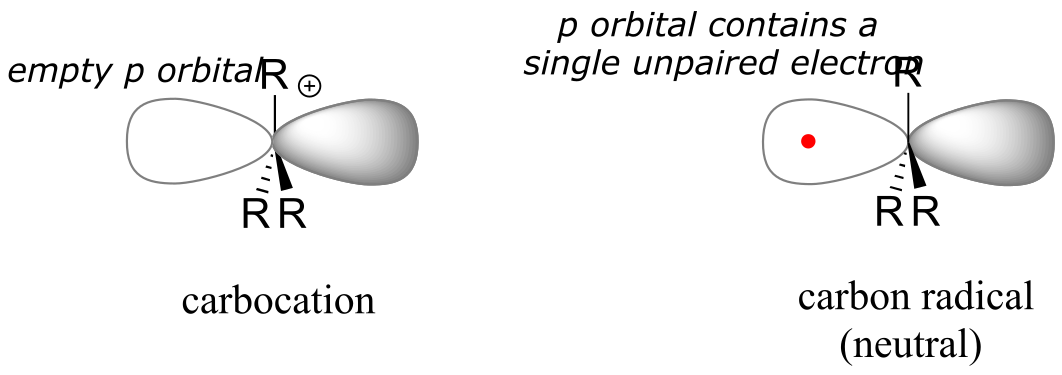
fig 5
When we studied electrophilic reactions in chapter 14, a major concern when evaluating possible mechanisms was the stability of any carbocation intermediate(s). Likewise, the stability of proposed radical intermediates is of great importance when evaluating the likelihood of possible single-electron mechanisms. Fortunately, the trend in the stability of carbon radicals parallels that of carbocations (section 8.5): tertiary radicals, for example, are more stable than secondary radicals, followed by primary and methyl radicals. This should make intuitive sense, because radicals, like carbocations, are electron deficient, and thus are stabilized by the electron-donating effects of nearby alkyl groups.
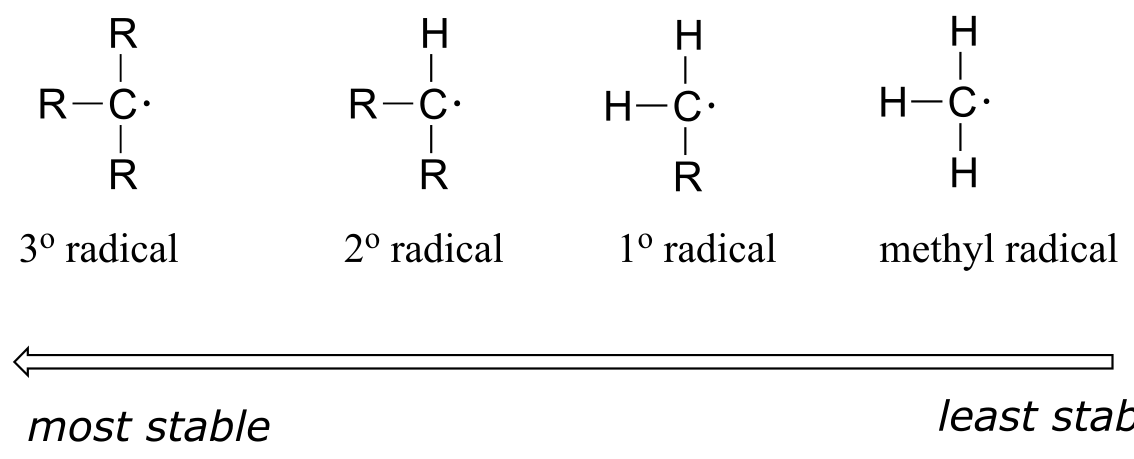
fig 6
Benzylic and allylic radicals are more stable than alkyl radicals due to resonance effects - an unpaired electron (just like a positive or negative charge) can be delocalized over a system of conjugated π bonds. An allylic radical, for example, can be pictured as a system of three parallel p orbitals sharing three electrons.

fig 7
The drawing below shows how a benzylic radical is delocalized to three additional carbons around the aromatic ring:
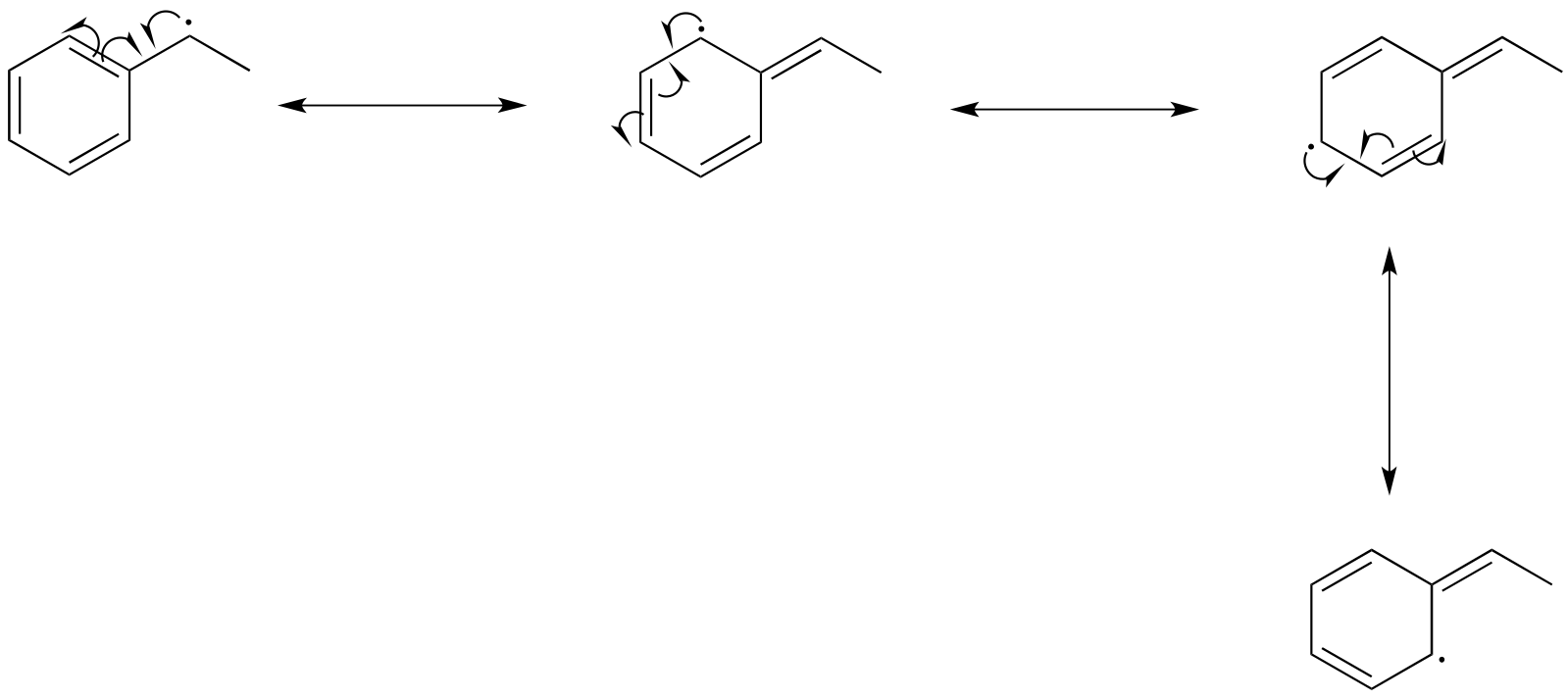
fig 8
Exercise 16.1: Just as phenolate ions are less reactive (less basic) than alkoxide ions, phenolic radicals are less reactive than alkoxide radicals. Draw one resonance contributor of a phenolic radical showing how the radical electron is delocalized to a ring carbon. Include electron-movement arrows.
While radical species are almost always very reactive and short-lived, in some extreme cases they can be unreactive. One example of an inert organic radical structure is shown below.
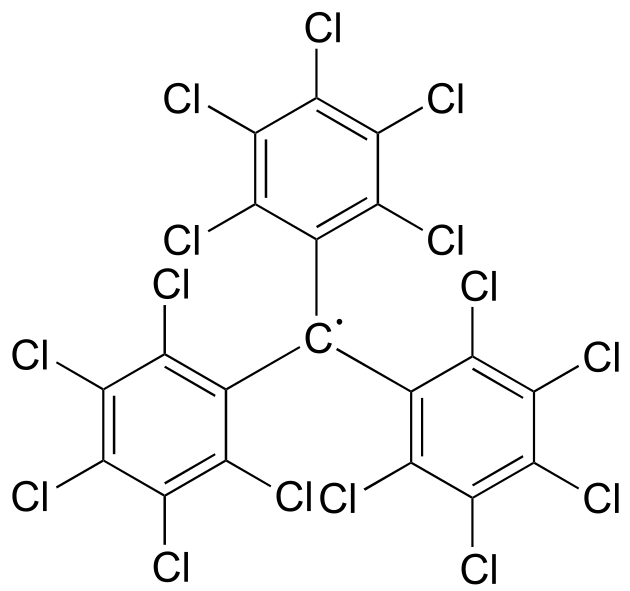
fig 10
The already extensive benzylic resonance stabilization is further enhanced by the fact that the large electron clouds on the chlorine atoms shield the radical center from external reagents. The radical is, in some sense, inside a protective ‘cage’.
Exercise 16.2: Draw a resonance contributor of the structure above in which the unpaired electron is formally located on a chlorine atom (include electron movement arrows)
Section 16.2: Radical chain reactions#
Because of their high reactivity, free radicals have the potential to be extremely powerful chemical tools - but as we will see in this chapter, they can also be extremely harmful in a biological/environmental context. Key to understanding many types of radical reactions is the idea of a radical chain reaction.
Radical chain reactions have three distinct phases: initiation, propagation, and termination. We’ll use a well-known example, the halogenation of an alkane such as ethane, to illustrate. The overall reaction is:
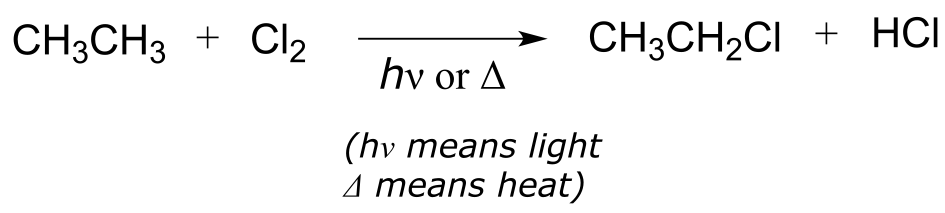
fig 11
The initiation phase in a radical chain reaction involves the homolytic cleavage of a weak single bond in a non-radical compound, resulting in two radical species as products.
Often, heat or light provides the energy necessary to overcome an energy barrier for this type of event. The initiation step in alkane halogenation is homolysis of molecular chlorine (Cl2) into two chlorine radicals. Keep in mind that that virtually all radical species, chlorine radicals included, are highly reactive.
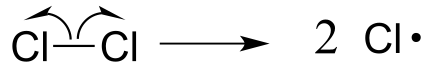
fig12
The propagation phase is the ‘chain’ part of chain reactions.
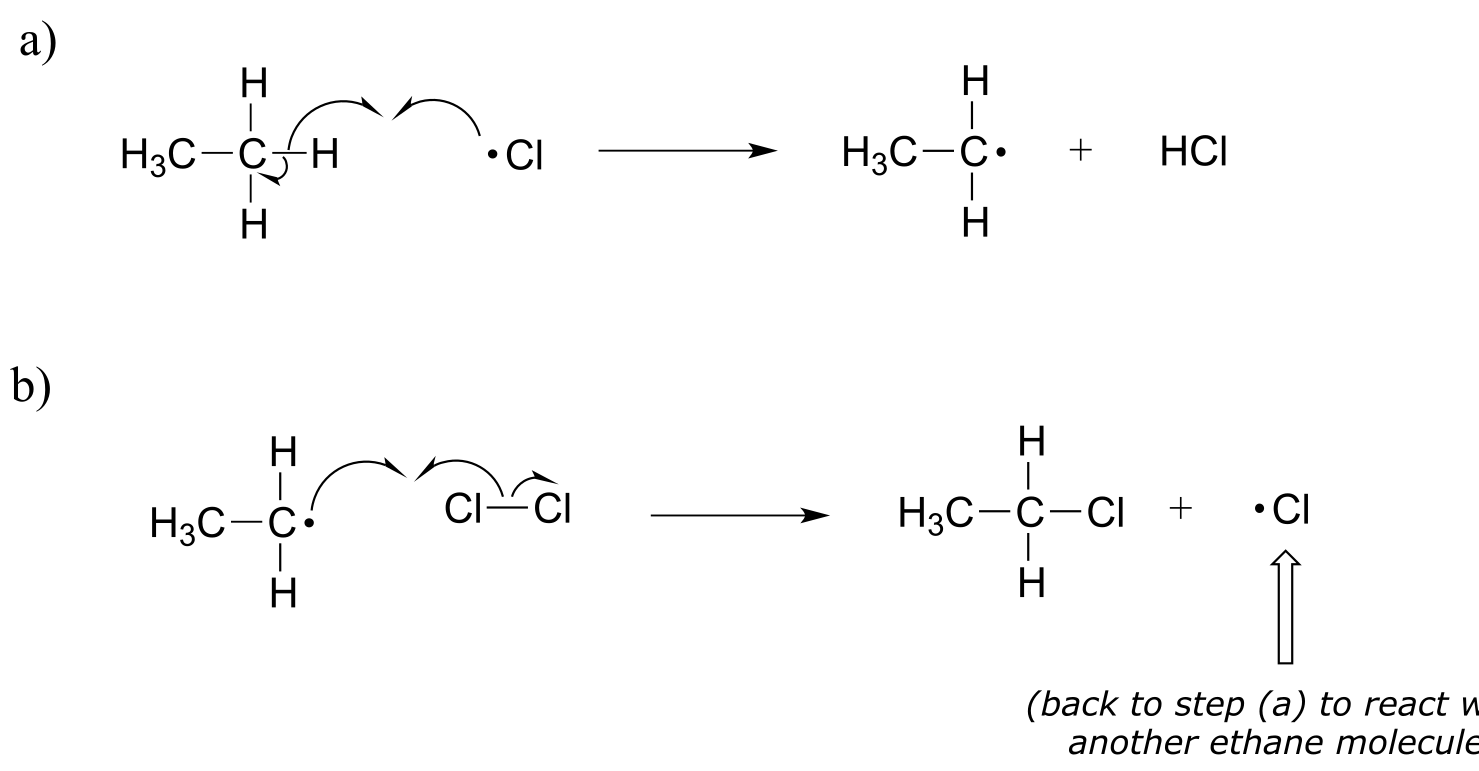
fig 13
Once a reactive free radical (chlorine radical in our example) is generated in the initiation phase, it will react with relatively stable, non-radical compounds to form a new radical species. In ethane halogenation, a chlorine radical generated in the initiation step first reacts with ethane in a hydrogen abstraction step, generating HCl and an ethyl radical (part a above). Then, the ethyl radical reacts with another (non-radical) Cl2 molecule, forming the chloroethane product and regenerating a chlorine radical (part b above). This process repeats itself again and again, as chlorine radicals formed in part (b) react with additional ethane molecules as in part (a).
The termination phase is a radical combination step, where two radical species happen to collide and react with each other to form a non-radical product and ‘break the chain’. In our ethane chlorination example, one possible termination event is the reaction of a chlorine radical with an ethyl radical to form chloroethane.
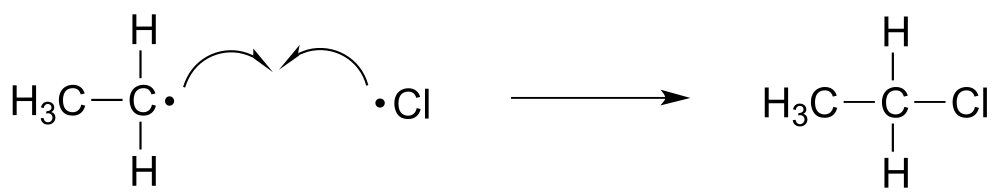
fig 14
Exercise 16.3: Draw two alternative chain termination steps in the ethane chlorination chain reaction. Which one leads to an undesired product?
Because radical species are so reactive and short-lived, their concentration in the reaction mixture at any given time is very low compared to the non-radical components such as ethane and Cl2. Thus, many cycles of the chain typically occur before a termination event takes place. In other words, a single initiation event leads to the formation of many product molecules.
Compounds which readily undergo homolytic cleavage to generate radicals are called radical initiators. As we have just seen, molecular chlorine and bromine will readily undergo homolytic cleavage to form radicals when subjected to heat or light. Other commonly used as radical initiators are peroxides and N-bromosuccinimide (NBS).
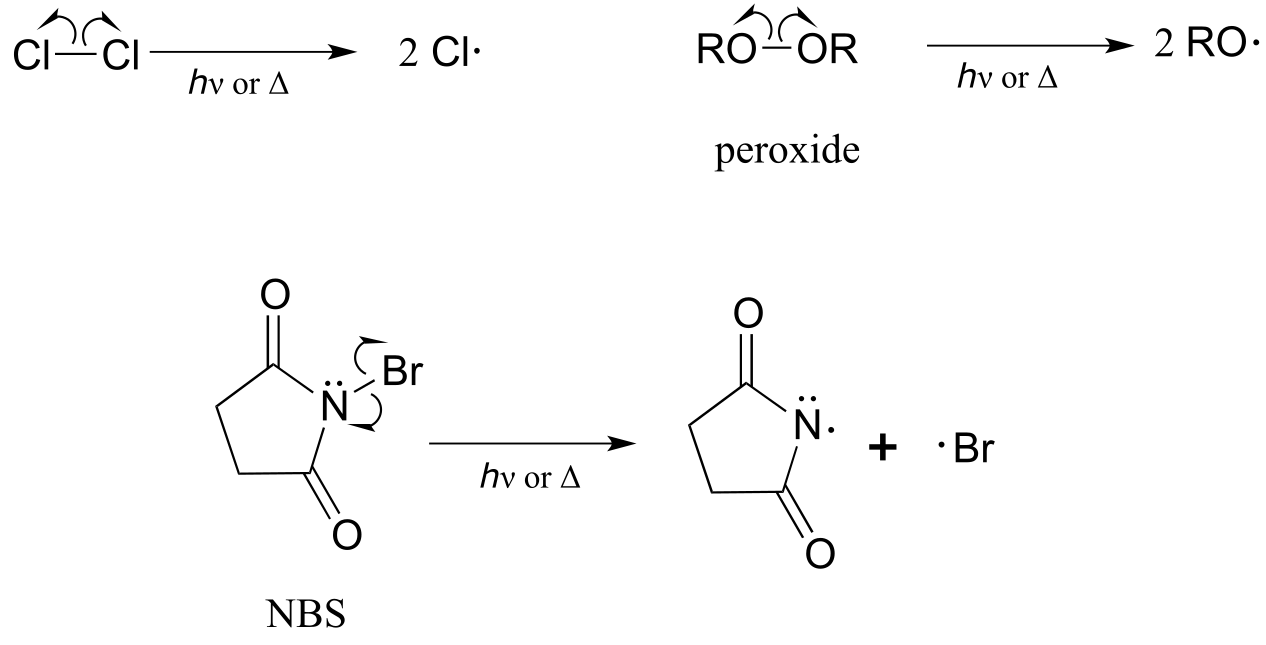
fig 15
Section 16.3: Useful polymers formed by radical chain reactions#
Many familiar household materials polymers made from radical chain reaction processes. Polyethylene (PET), the plastic material used to make soft drink bottles and many other kinds of packaging, is produced by the radical polymerization of ethylene (ethylene is a common name for what we call ‘ethene’ in IUPAC nomenclature). The process begins when a radical initiator such as benzoyl peroxide undergoes homolytic cleavage at high temperature:
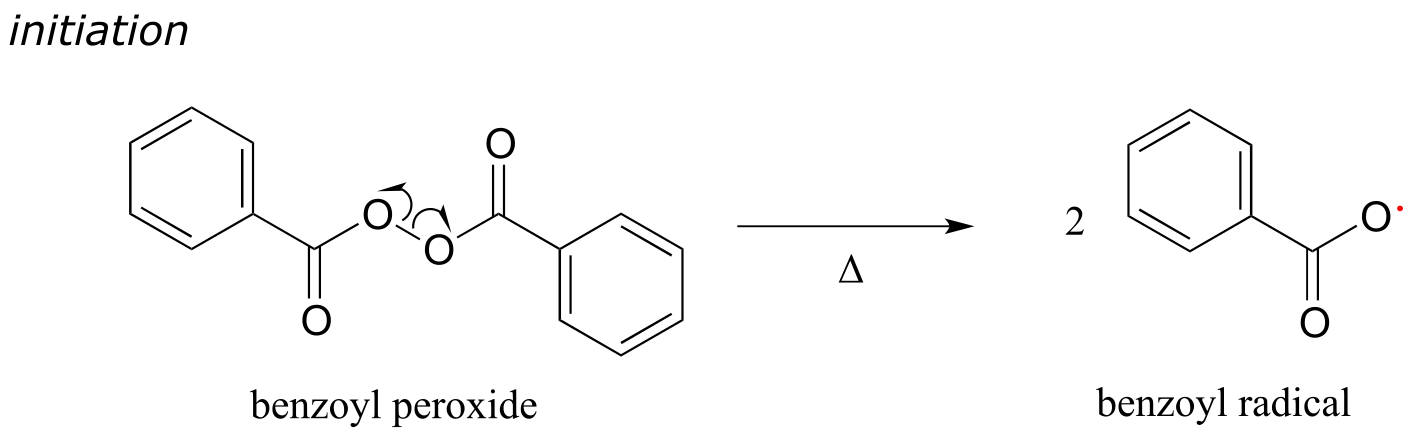
fig 16
In the propagation phase, the benzoyl radical (X• in the figure below) adds to the double bond of ethylene, generating a new organic radical.
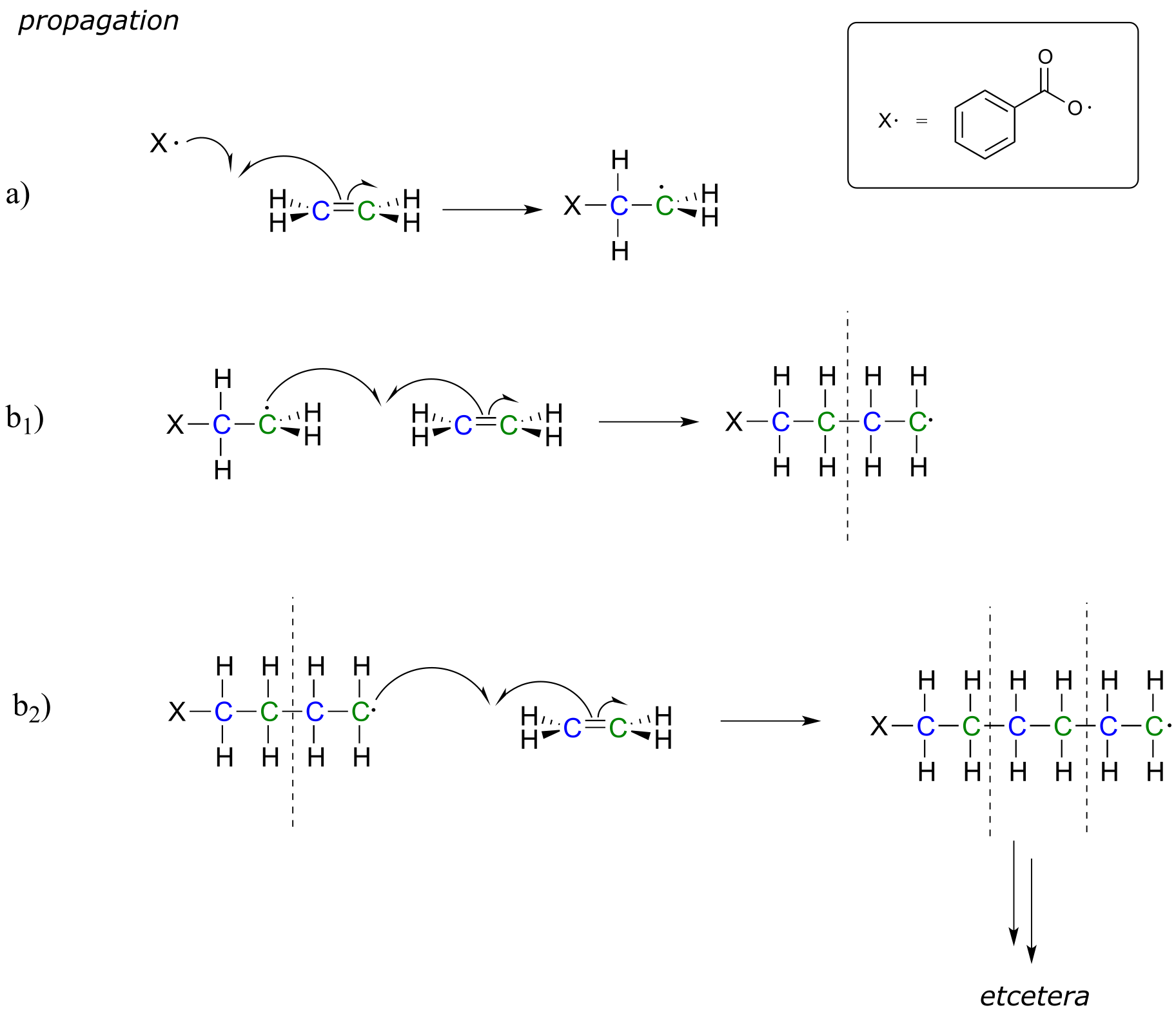
fig 17
Successive ethylene molecules add to the growing polymer, until termination occurs when two radicals happen to collide.
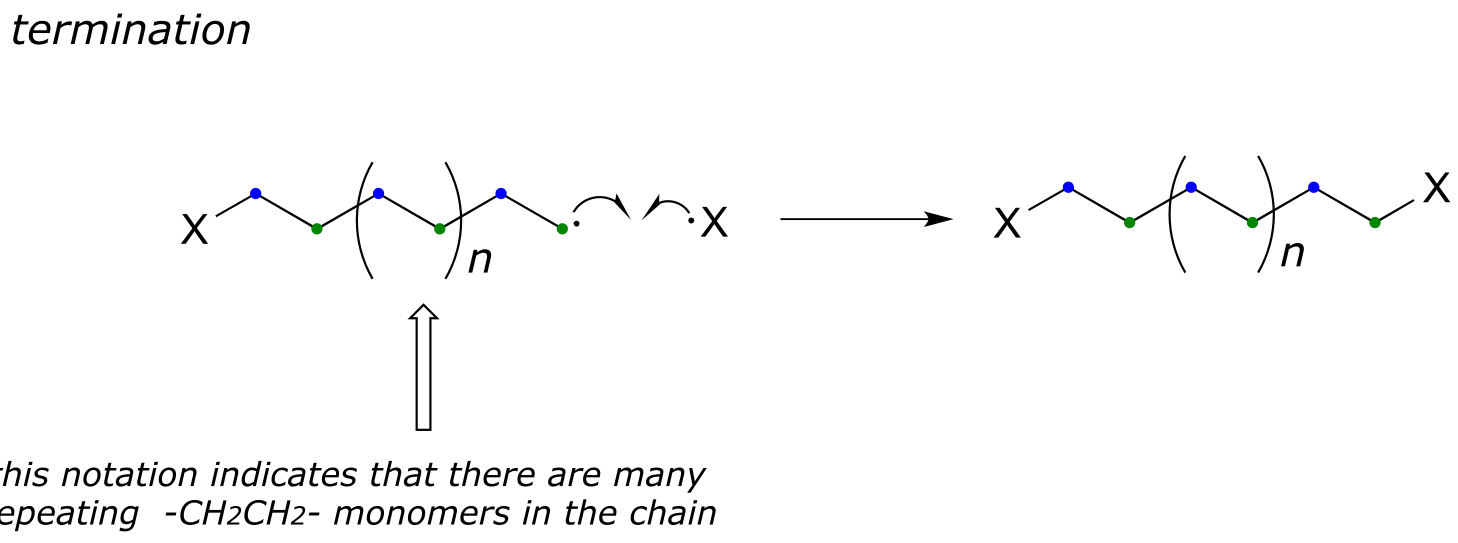
fig 18
The length of the polymer is governed by how long the propagation phase continues before termination, and can usually be controlled by adjusting reaction conditions.
Other small substituted alkene monomers polymerize in a similar fashion to form familiar polymer materials. Two examples are given below.
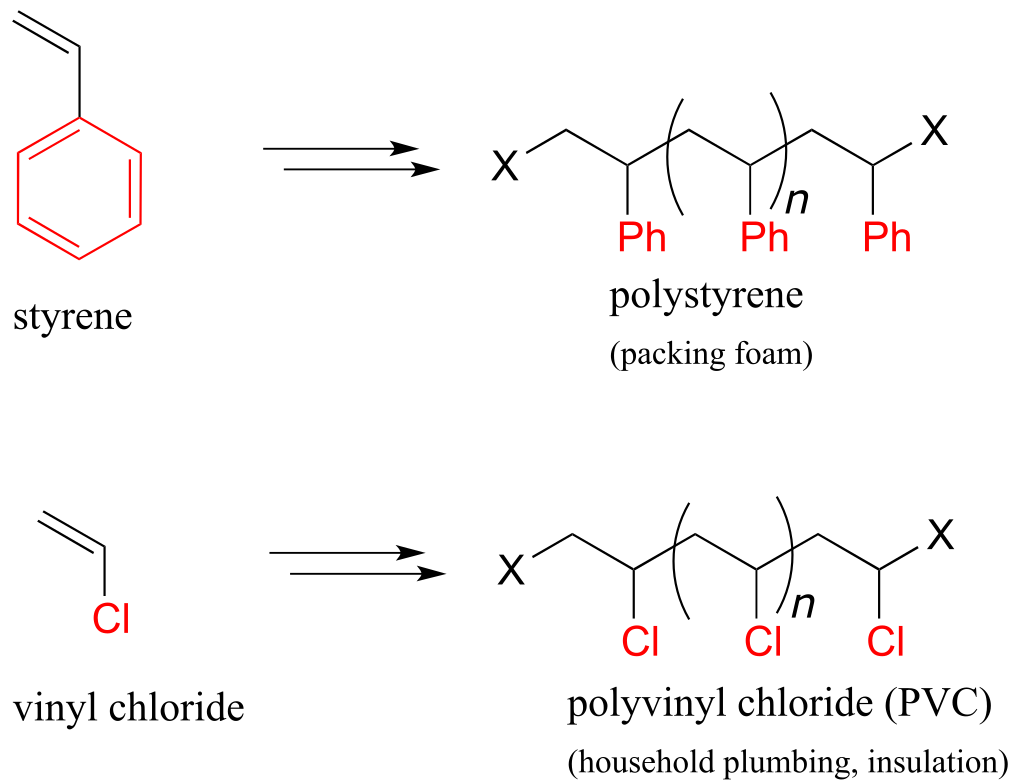
fig 19
Exercise 16.4: Show a mechanism for the formation of a 2-unit long section of polystyrene, starting with the monomer and benzoyl peroxide initiator. Keep in mind the relative stability of different radical intermediates.
A common way to separate proteins in the biochemistry lab is through a technique called polyacrylamide gel electrophoresis (PAGE). The polyacrylamide gel is formed through radical polymerization of acrylamide monomer, with the ammonium salt of persulfate used as the radical initiator.
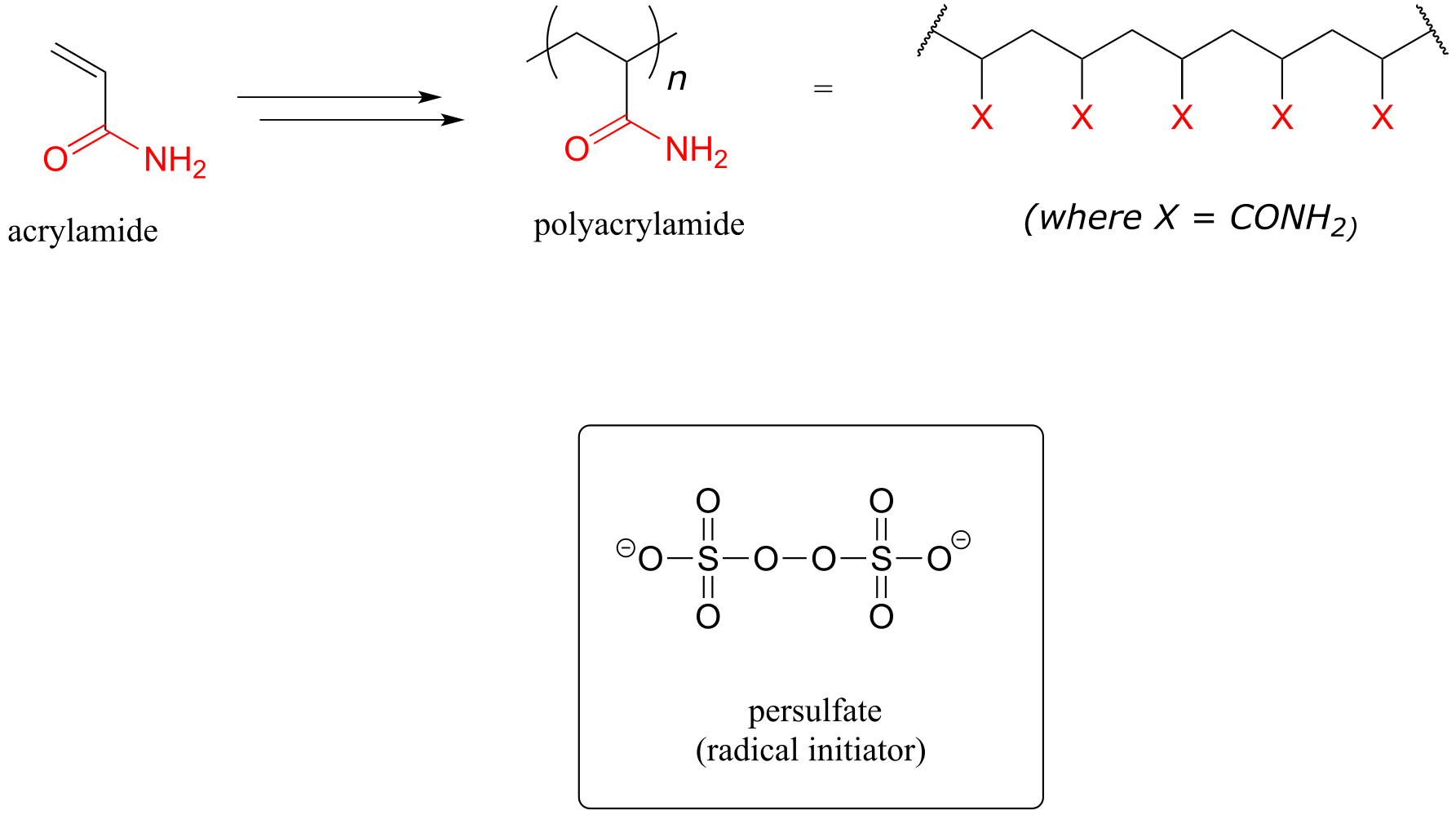
fig 20
In the end of chapter problems, you will be invited to propose a mechanism showing how a molecule called ‘bis-acrylamide’ serves as a ‘crosslinker’ between linear polyacrylamide chains to allow for formation of a net-like structure for the PAGE gel.
Section 16.4: Destruction of the ozone layer by a radical chain reaction#
The high reactivity of free radicals and the multiplicative nature of radical chain reactions can be useful in the synthesis of materials such as polyethylene plastic - but these same factors can also result in dangerous consequences in a biological or ecological context. You are probably aware of the danger posed to the earth’s protective stratospheric ozone layer by the use of chlorofluorocarbons (CFCs) as refrigerants and propellants in aerosol spray cans. Freon-11, or CFCl3, is a typical CFC that was widely used until late in the 20th century. It can take months or years for a CFC molecule to drift up into the stratosphere from the surface of the earth, and of course the concentration of CFCs at this altitude is very low. Ozone, on the other hand, is continually being formed in the stratosphere. Why all the concern, then, about destruction of the ozone layer - how could such a small amount of CFCs possibly do significant damage? The problem lies in the fact that the process by which ozone is destroyed is a chain reaction, so that a single CFC molecule can initiate the destruction of many ozone molecules before a chain termination event occurs.
Although there are several different processes by which the ozone destruction process might occur, the most important is believed to be the chain reaction shown below.
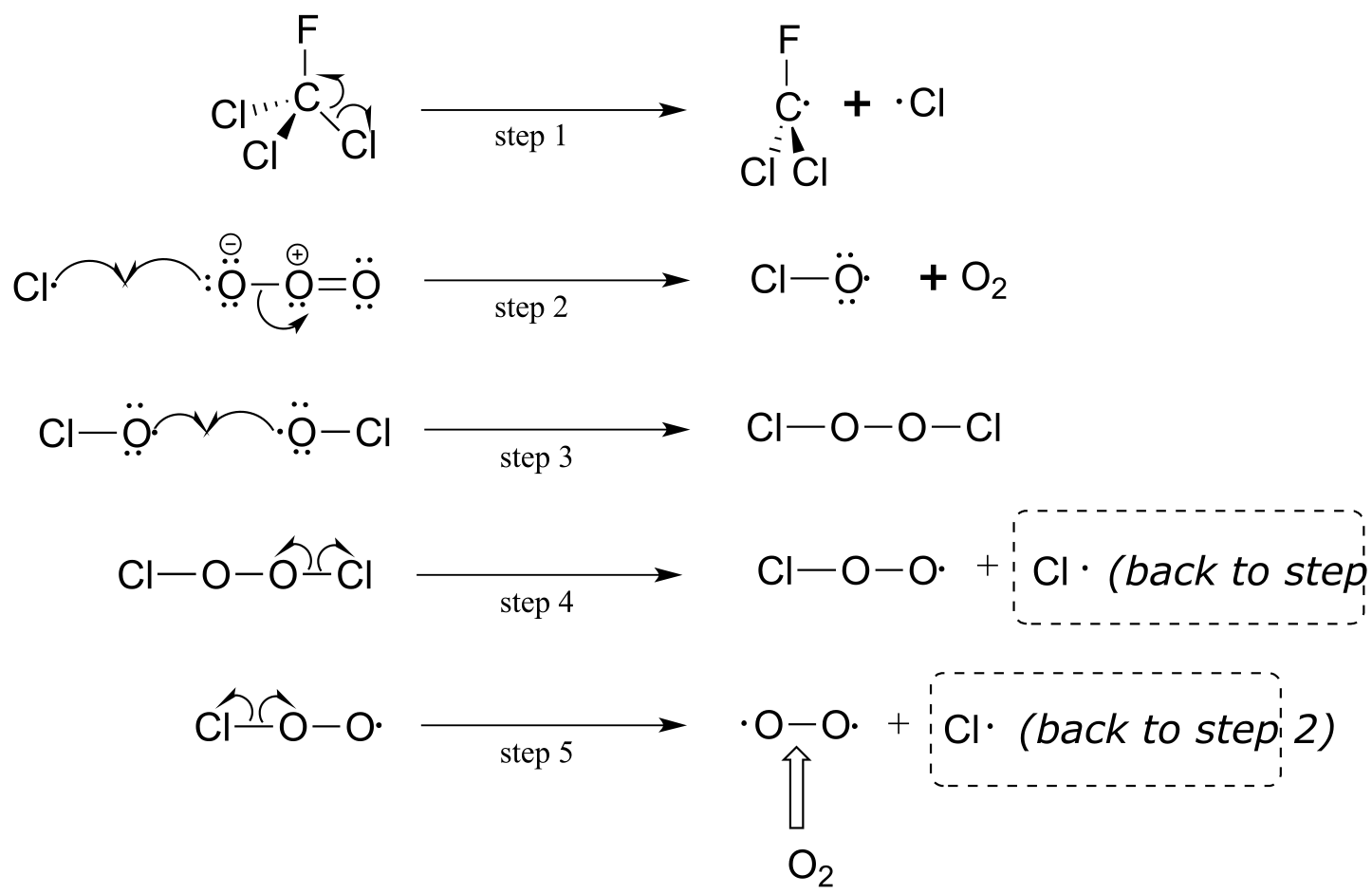
fig 21
First, a CFC molecule undergoes homolytic cleavage upon exposure to UV radiation, resulting in the formation of two radicals (step 1). The chlorine radical rapidly reacts with ozone (step 2) to form molecular oxygen and a chlorine monoxide radical. Step 3 appears to be a chain termination step, as two chlorine monoxide radicals combine. The Cl2O2 condensation product, however, is highly reactive and undergoes two successive homolytic cleavage events (steps 4 and 5) to form O2 and two chlorine radicals, which propagates the chain.
To address the problem of ozone destruction, materials chemists have developed new hydrofluorocarbon refrigerant compounds. The newer compounds contain carbon-hydrogen bonds, which are weaker than the carbon-halogen bonds in CFCs , and thus are susceptible to homolytic cleavage caused by small amounts of hydroxide radical present in the lower atmosphere:
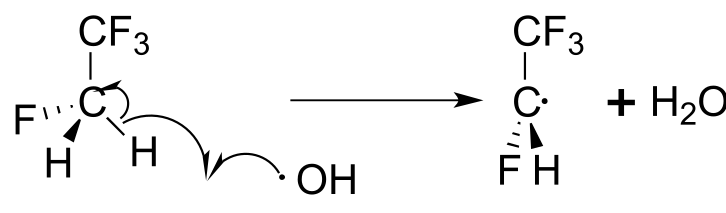
fig 22
This degradation occurs before the refrigerant molecules have a chance to drift higher up to the stratosphere where the ozone plays its important protective role. The degradation products are quite unstable and quickly degrade further into relatively harmless by-products. The hydroxide radical is sometimes referred to as an atmospheric ‘detergent’ due to its ability to degrade escaped refrigerants and other volatile organic pollutants.
Hydrofluorocarbons do, however, act as greenhouse gases, and are thought to contribute to climate change.
Section 16.5: Oxidative damage, vitamin C, and scurvy#
While the hydroxide radical can be a beneficial ‘detergent’ in the atmosphere, it is harmful when present in a living cell. Hydroxide radical is one of the reactive oxygen species (ROS) that we learned about in chapter 15. Recall that ROS are continuously produced as minor but harmful side-products in the reduction of O2 to H2O in respiration.
You may recall from your general chemistry course that molecular oxygen exists in two states: ‘singlet’ oxygen has a double bond and no unpaired electrons, while ‘triplet’ oxygen has a single O-O bond and two unpaired electrons. Molecular orbital theory - and experimental evidence - show that the triplet state is lower in energy.

fig 23
ROS are highly reactive oxidizing agents, capable of inflicting damage to DNA, proteins, and the lipids of cell membranes - they are thought to play a major role in the aging process. Hydroxide radical, for example, can initiate a radical chain reaction with the hydrocarbon chain of an unsaturated membrane lipid molecule, resulting in the formation of lipid hydroperoxide.
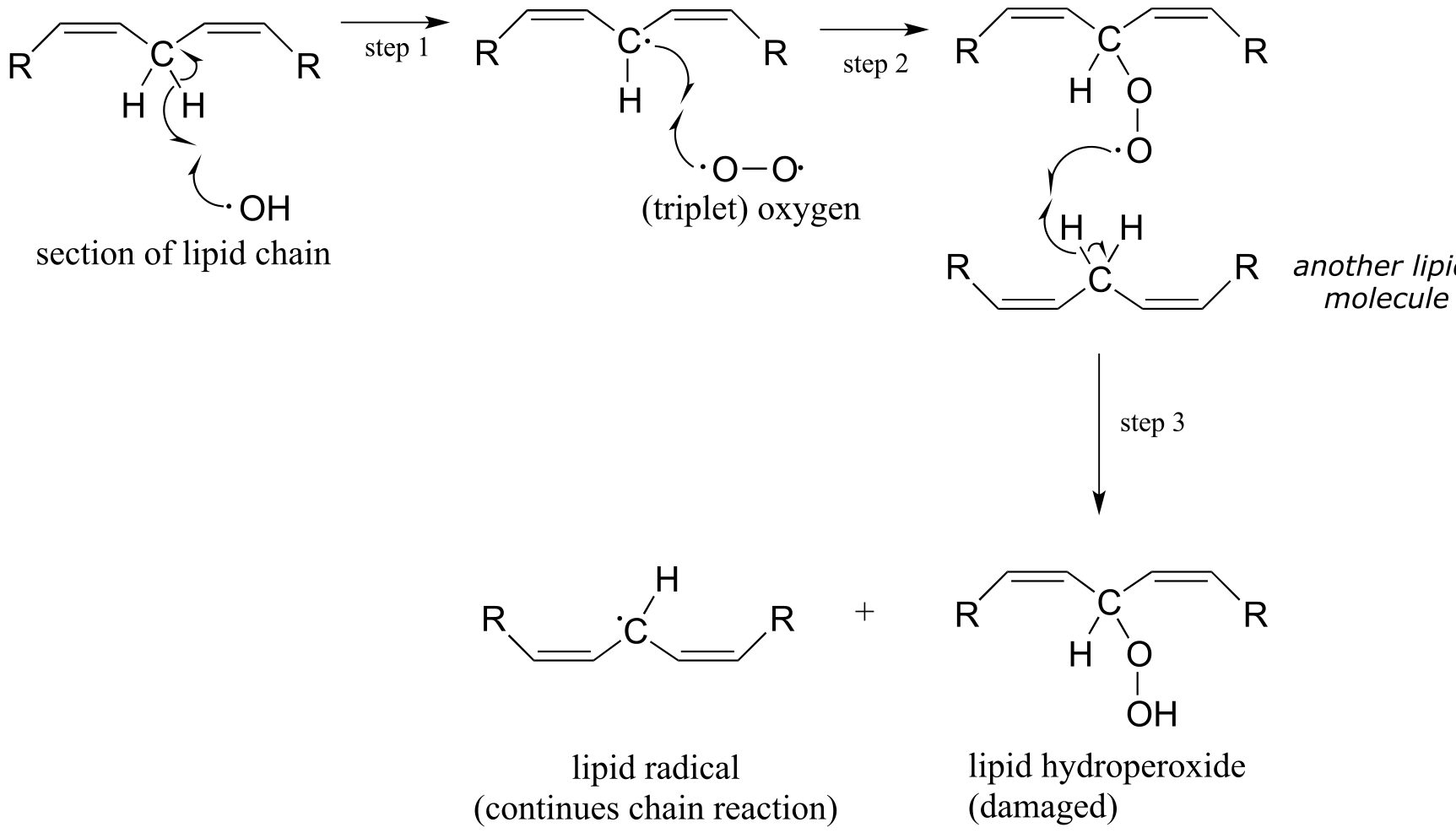
fig 24
The allylic lipid radical formed as the result of homolytic hydrogen abstraction by hydroxide radical (step 1 above) reacts with one of the unpaired electrons in triplet oxygen (step 2) forming a peroxy radical. This radical species in turn homolytically abstracts a hydrogen from another lipid molecule (step 3), thus propagating the chain.
Many edible plants contain various antioxidant compounds, also known as ‘free radical scavengers’, which serve to protect cells from the oxidative effects of hydroxide radical and other harmful radical intermediates. Simply put, a free radical scavenger is a molecule that reacts with a potentially damaging free radical species, forming a more stable radical species which can be metabolized by the body before any damage is done to cell constituents.
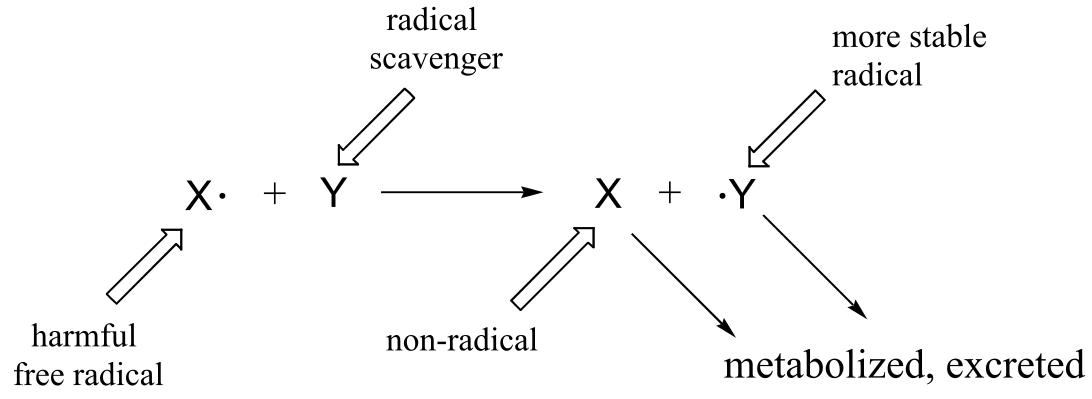
In the introduction to this chapter, we learned about scurvy, the disease long dreaded by sailors, and how it is caused by a deficiency of ascorbic acid (vitamin C) in the diet. We will soon get to the connection between ascorbic acid and scurvy, but first, let’s look at how ascorbic acid functions as a free radical scavenger in your body.
The pKa of ascorbic acid is about 4.1, so in a physiological environment it exists mainly as ascorbate anion, the conjugate base. When ascorbate encounters a hydroxide radical (or any other potentially damaging radical species), it donates a single electron, thus reducing the hydroxide radical to hydroxide ion and becoming itself an ascorbyl radical.
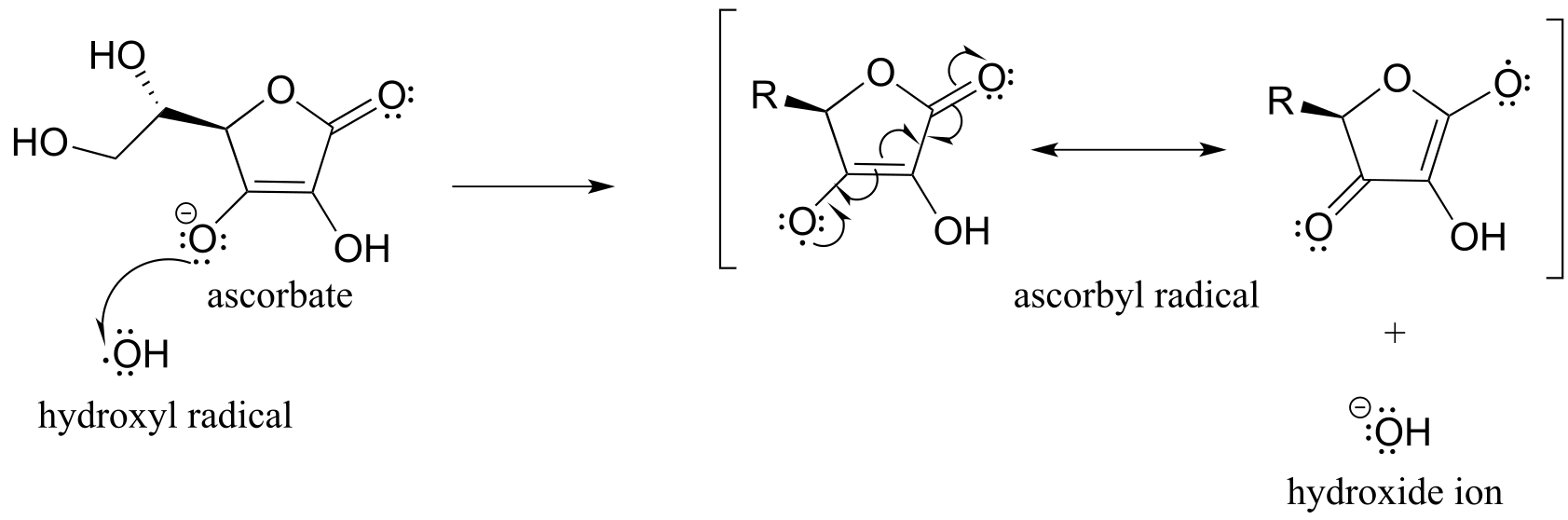
fig 24a
The ascorbyl radical is stabilized by resonance. The end result of this first step is that a very reactive, potentially harmful hydroxide radical has been ‘quenched’ to hydroxide ion and replaced by a much less reactive (and thus less harmful) ascorbyl radical.
The ascorbyl radical can then donate a second electron to quench a second hydroxide radical, resulting in the formation of dehydroascorbate, the oxidized form of ascorbate.
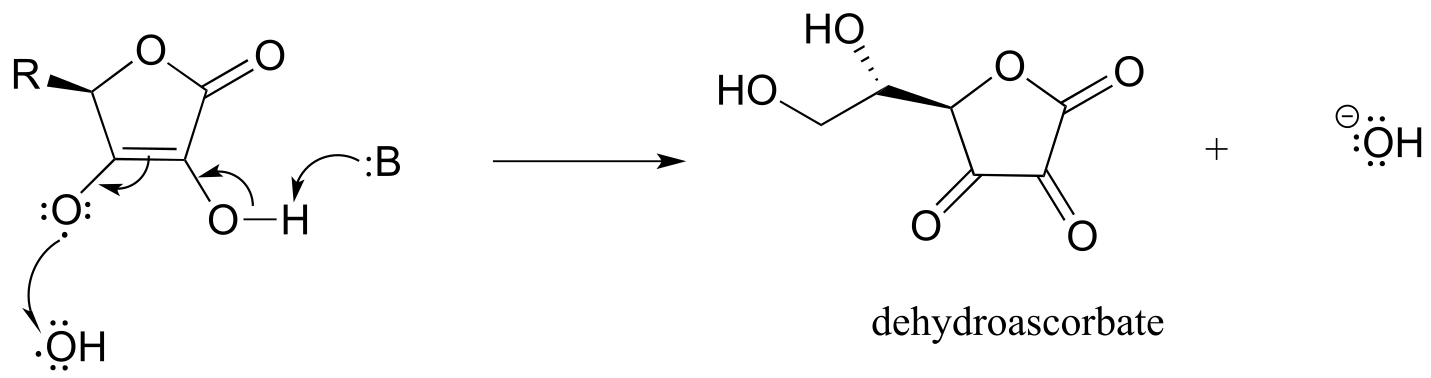
fig 24b
One ascorbate molecule is thus potentially able to scavenge two harmful radical species.
Dehydroascorbate is subsequently either broken down and excreted, or else enzymatically recycled (reduced) back to ascorbic acid. You were invited to propose a mechanism for the latter (redox) step in problem 15.10. J. Am. Coll. Nutr., 2003, 22, 18
We learned in the introduction to this chapter about the gruesome effects of long-term ascorbic acid deficiency. What, then, is the chemical connection between ascorbic acid and scurvy?
The symptoms association with scurvy are caused by the body’s failure to properly synthesize collagen, the primary structural protein in our connective tissues. Essential to the stability of collagen is its ability to form a unique triple-helical structure, in which three protein strands coil around each other like a woven rope. Collagen strands are not able to pack together properly into their triple helix structure unless certain of their proline amino acid residues are hydroxylated: the electronegative OH group on hydroxyproline causes the five-membered ring in the amino acid to favor a particular ‘envelope’ conformation (section 3.2) as well as the ‘trans’ peptide conformation, both of which are necessary for stable triple-helix formation.
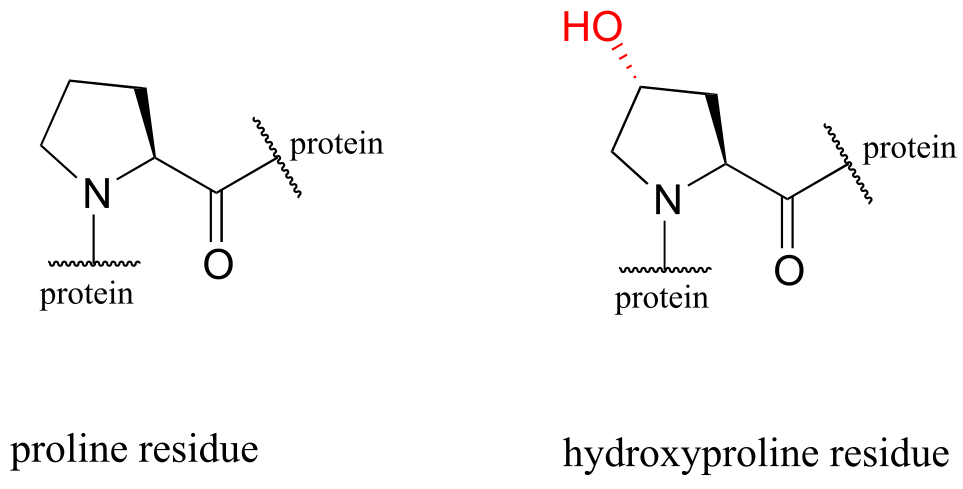
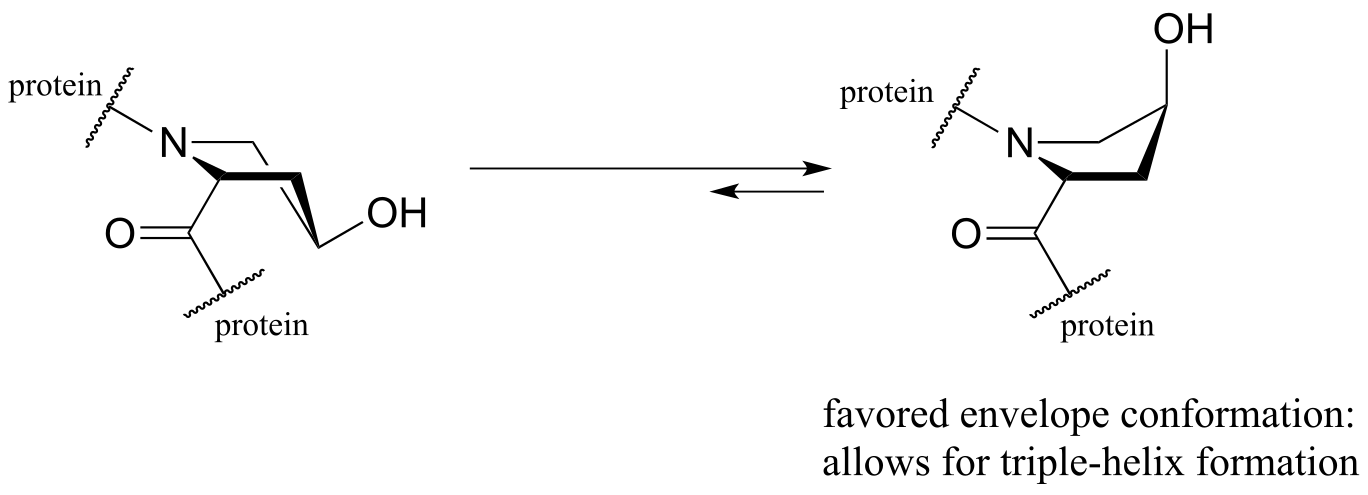

fig 26a
Proline hydroxylase, the enzyme responsible for this key modification reaction, depends in turn upon the presence of ascorbate. The hydroxylating reaction is complex, and involves electron-transfer steps with enzyme-bound iron - mechanistic details that are well outside of our scope here, but which you may learn about in a bioinorganic chemistry course. It is enough for us to know that iron starts out in the Fe+2 state, and during the course of the reaction it loses an electron to assume the Fe+3 state. In order for the enzyme to catalyze another reaction, the iron must be reduced back to its active Fe+2 state - it must accept a single electron. The donor of this single electron is ascorbate.
*(*For more information, see Crit. Rev. Biochem. Mol. Biol. 2010, 405, 106.)
So, to sum up: If we fail to get enough ascorbic acid in our diet (in other words, if we don’t eat our fruits and vegetables!) the iron in our proline hydroxylase enzymes won’t be returned to the active Fe+2 state, so the catalytic cycle is broken and we can’t turn prolines into hydroxyprolines. Without the hydroxy group, the proline residues of our collagen proteins won’t assume the proper conformation, and as a consequence the collagen triple helix structures will be unstable. At physiological temperature, our collagen will literally melt apart - and with it, our gums, our capillaries, and anything else held together by connective tissue. This is scurvy.
You have probably heard that many fruits and vegetables contain natural ‘polyphenol’ antioxidant compounds that are thought to be beneficial to our health. Apigenin, for example, is found in parsley and celery, while the skins of grapes used to produce red wine are particularly rich in resveratrol, as well as many other polyphenols. Curcumin is the compound responsible for the distinctive yellow color of turmeric, a ubiquitous spice in Indian cuisine.
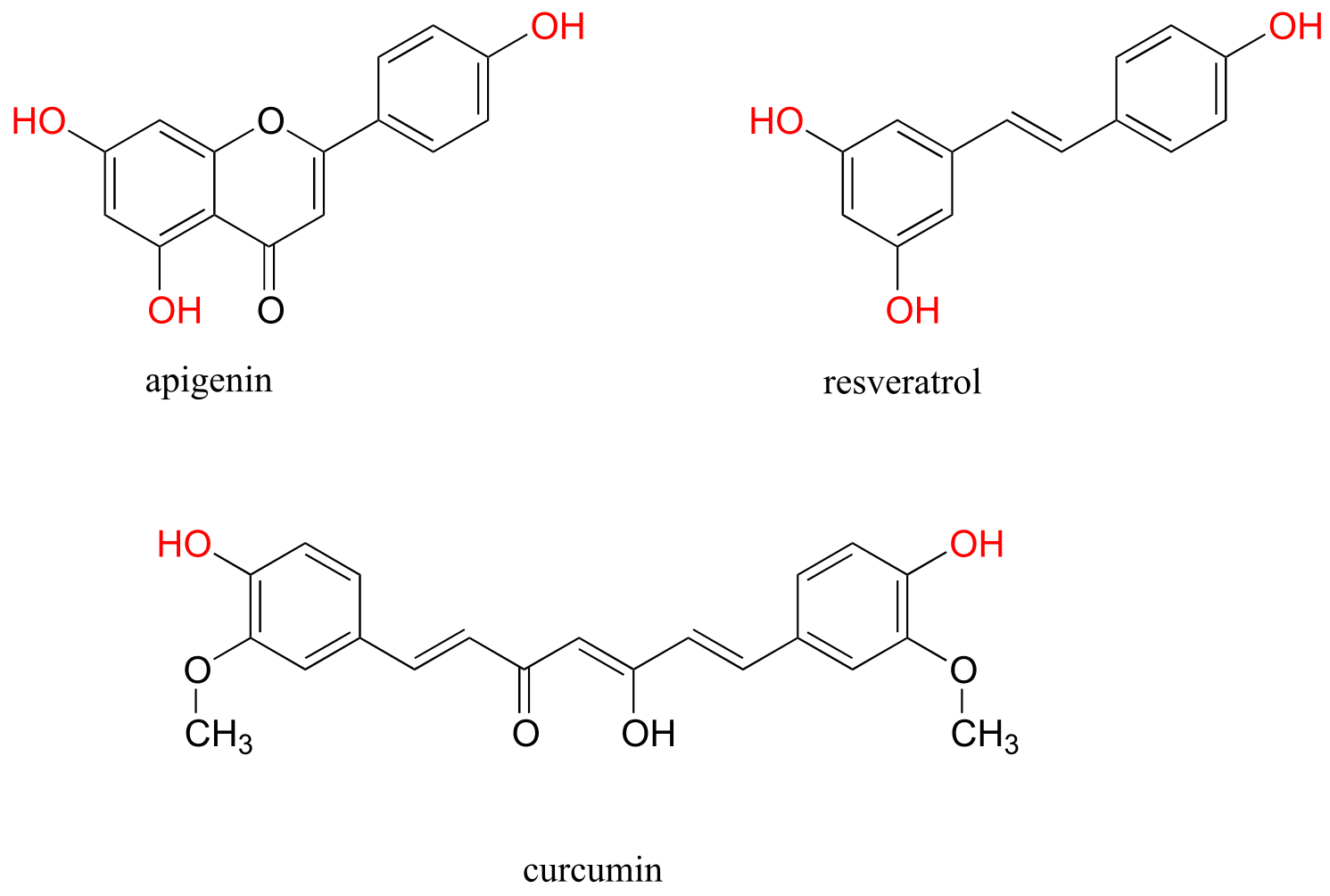
fig 25
While much remains to be learned about exactly how these polyphenols exert their antioxidant effects, it is likely that they, like ascorbic acid, act as radical scavengers. For example, resveratrol could donate a single electron (and a proton) to hydroxide radical to reduce it to water. The phenolic radical that results is stabilized by resonance, and is much less likely than hydroxide radical to cause damage to important biomolecules in the cell.
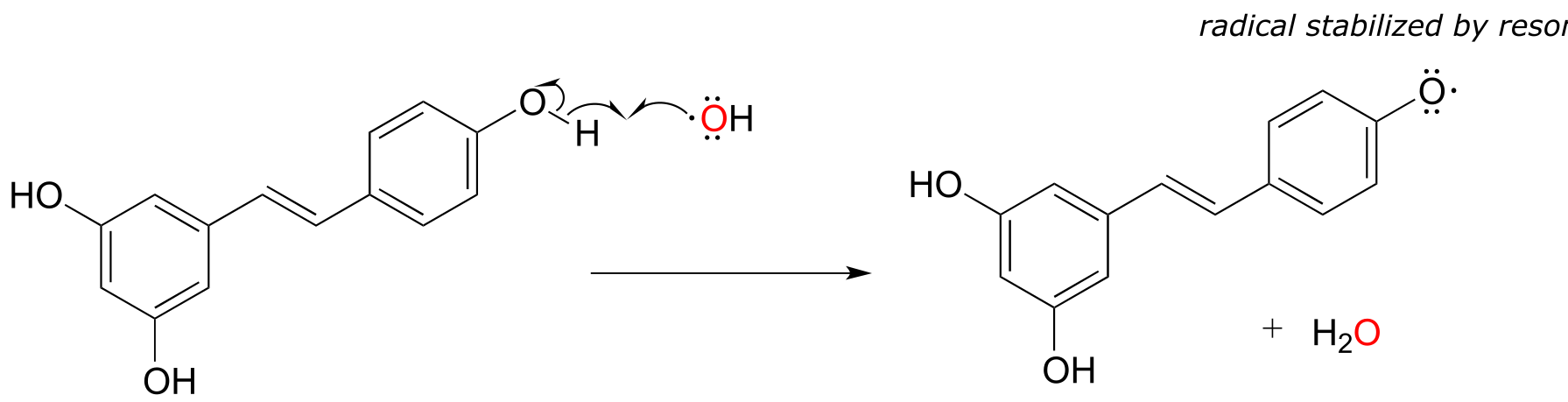
fig 26
Section 16.6: Flavin as a single-electron carrier#
In chapter 15 we saw how a nicotinamide and flavin coenzymes can act as acceptors or donors of two electrons in hydride-transfer redox steps. Recall that it was mentioned that flavin, (but not nicotinamide) can also participate in single-electron transfer steps through a stabilized radical intermediate called a semiquinone.. Frey p. 162 fig 3-30; Silverman p. 122 sch. 3.34; J Phys Chem A. 2013, 117, 11136 fig 2)
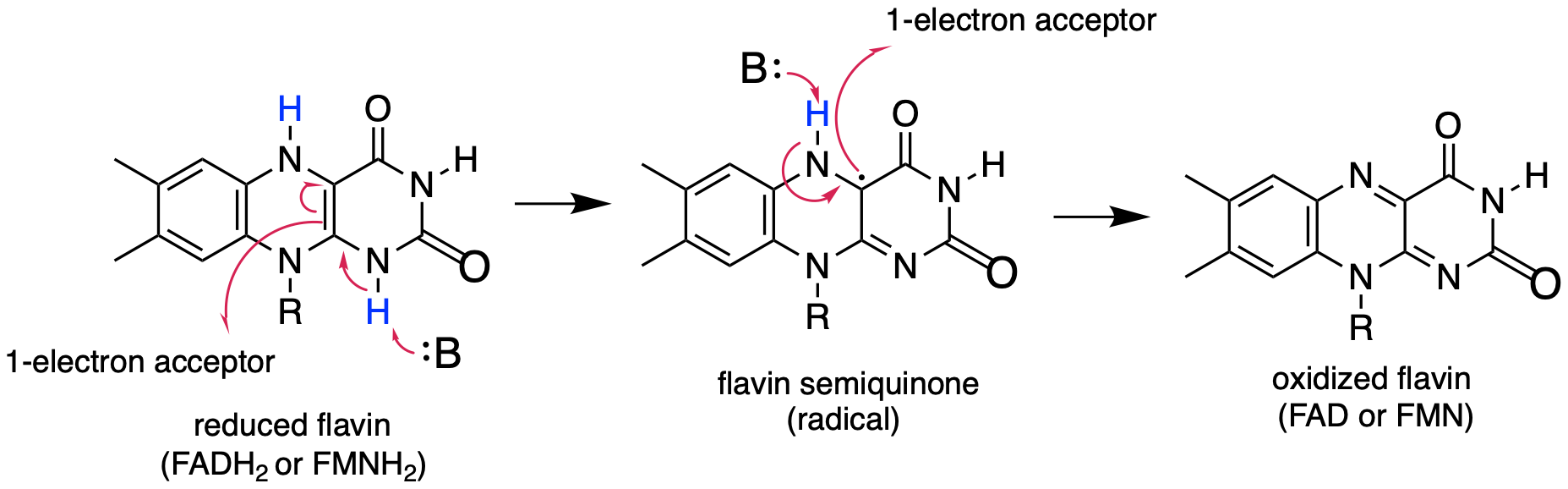
fig 27
Note in this reaction that overall, flavin loses or gains two electrons and two protons, just like in the flavin-dependent redox reactions we saw in chapter 15. The difference here is that the electrons are transferred one at a time, rather than paired in the form of a hydride ion.
Two important examples single-electron acceptor species in human metabolism are ubiquinone (coenzyme Q) and the oxidized form of cytochrome. Ubiquinone is a coenzyme that can transfer single electrons via a semiquinone state analogous to that of flavin, and cytochrome is a protein containing a ‘heme’ iron center which shuttles between the Fe+3 (oxidized) and Fe+2 (reduced) state.
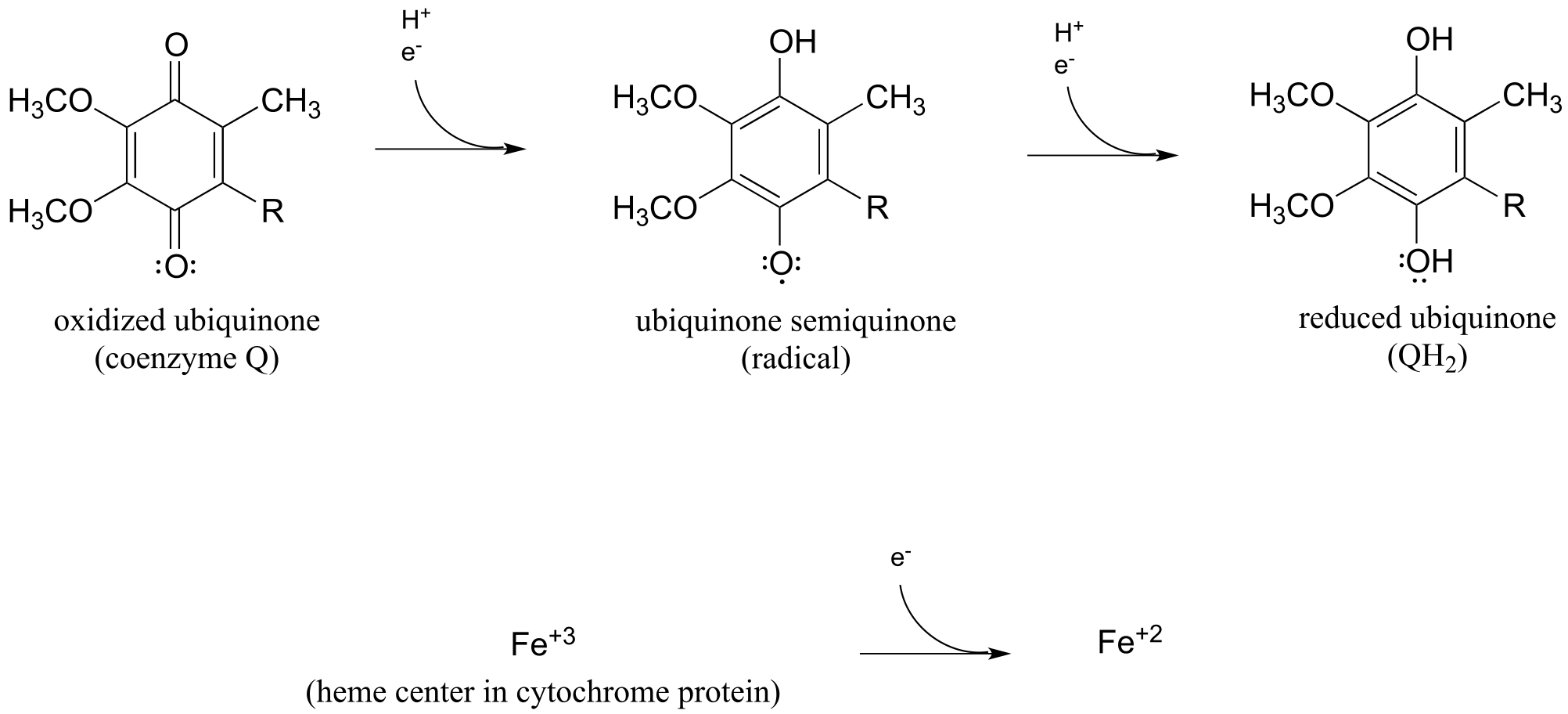
fig 27a
Further discussion of the mechanisms of single-electron flavin reactions is beyond our scope here, but when you study the ‘respiratory chain’ in a biochemistry course you will gain a deeper appreciation for the importance of flavin in single-electron transfer processes.
Problems#
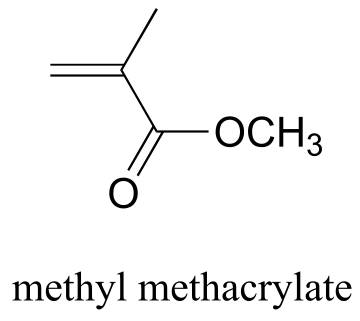
P16.1: Plexiglass is a polymer of methyl methacrylate. Show a mechanism for the first two propagation steps of polymerization (use X• to denote the radical initiator), and show a structure for the plexiglass polymer. Assume an alkene addition process similar to that shown in the text for polyethylene.
P16.2: In section 16.3 we saw how acrylamide polymerizes to form the polyacrylamide used in PAGE protein gels. Polyacrylamide by itself is not sufficient by itself to form the gel - the long polyacrylamide chains simply slip against each other, like boiled spaghetti. To make a PAGE gel, with pores for the proteins to slip through, we need a crosslinker - something to tie the chains together, forming a three-dimensional web-like structure. Usually, a small amount of bis-acrylamide is added to the acrylamide in the polymerization mixture for this purpose.
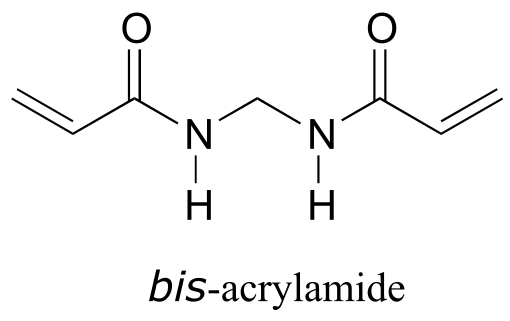
Propose a radical mechanism showing how bis- acrylamide might form crosslinks between two polyacrylamide chains.
P16.3: Resveratrol is a natural antioxidant found in red wine (see section 16.5 for the structure).
a) Draw one resonance structure to illustrate how the resveratrol radical is delocalized by resonance.
b) Indicate all of the carbons on your structure to which the radical can be delocalized.
c) Draw an alternate resveratrol radical (one in which a hydrogen atom from one of the other two phenolic groups has been abstracted). To how many carbons can this radical be delocalized?
d) The curcumin structure is shown in the same figure as that of resveratrol, in section16.5. Draw two resonance contributors of a curcumin radical, one in which the unpaired electron is on a phenolic oxygen, and one in which the unpaired electron is on a ketone oxygen.
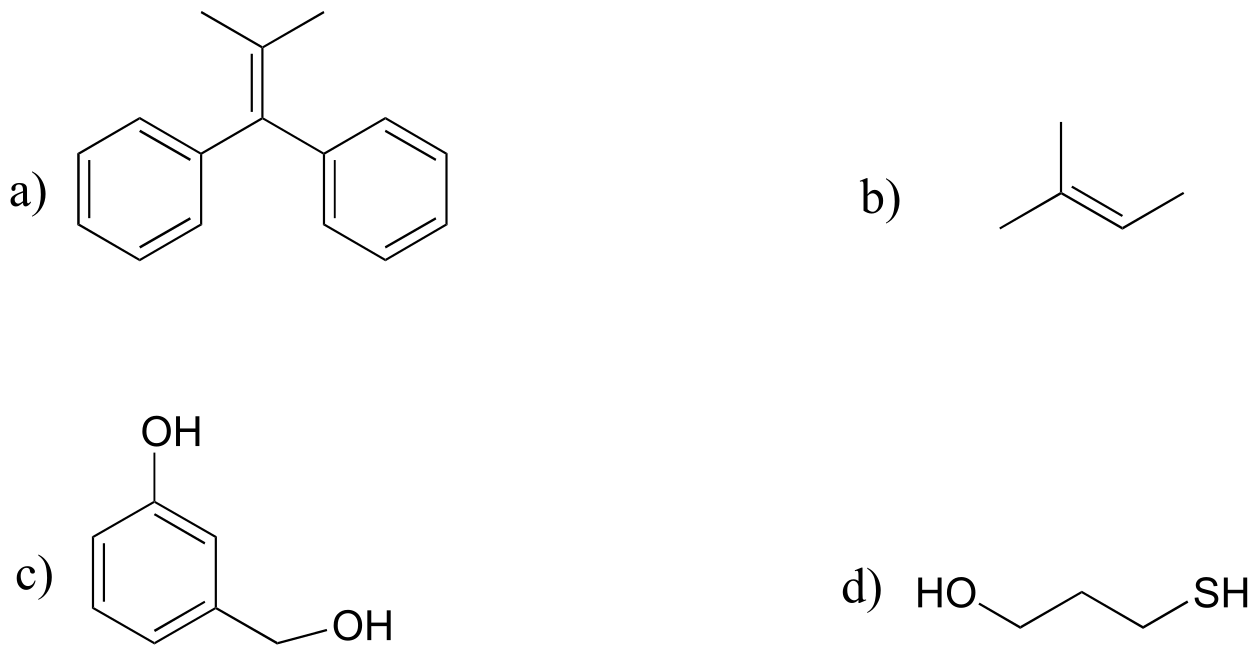
P16.4: Draw the radical intermediate species that you would expect to form when each of the compounds below reacts with a radical initiator.
P16.5: Azobis(isobutyronitrile) is a widely used radical initiator which rapidly undergoes homolytic decomposition when heated.
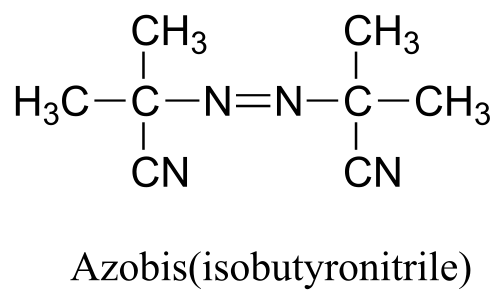
Predict the products of this decomposition reaction, and show a likely mechanism. What is the thermodynamic driving force for homolytic cleavage?
P16.6:
a) When 2-methylbutane is subjected to chlorine gas and heat, a number of isomeric chloroalkanes with the formula C5H11Cl can form. Draw structures for these isomers, and for each draw the alkyl radical intermediate that led to its formation.
b) In part a), which is the most stable radical intermediate?
c) In the reaction in part a), the relative abundance of different isomers in the product is not exclusively a reflection of the relative stability of radical intermediates. Explain.
P16.7: We learned in chapter 14 that HBr will react with alkenes in electrophilic addition reactions with ‘Markovnikov’ regioselectivity. However, when the starting alkene contains even a small amount of contaminating peroxide (which happens when it is allowed to come into contact with air), a significant amount of ‘anti-Markovnikov’ product is often observed.
a) Propose a mechanism for formation of the anti-Markovnikov addition product when 1-butene reacts with HBr containing a small amount of benzoyl peroxide
b) Predict the product and propose a mechanism for the addition of ethanethiol to 1-butene in the presence of peroxide.
P16.8: In section 11.5 we learned that aspirin works by blocking the action of an enzyme that catalyzes a key step in the biosynthesis of prostaglandins, a class of biochemical signaling molecules. The enzyme in question, prostaglandin H synthase (EC 1.14.99.1) catalyzes the reaction via several single-electron steps. First, an iron-bound oxygen radical in the enzyme abstracts a hydrogen atom from arachidonate. The arachidonate radical intermediate then reacts with molecular oxygen to form a five-membered oxygen-containing ring, followed by closure of a cyclopentane ring to yield yet another radical intermediate. (Biochemistry 2002, 41, 15451.)
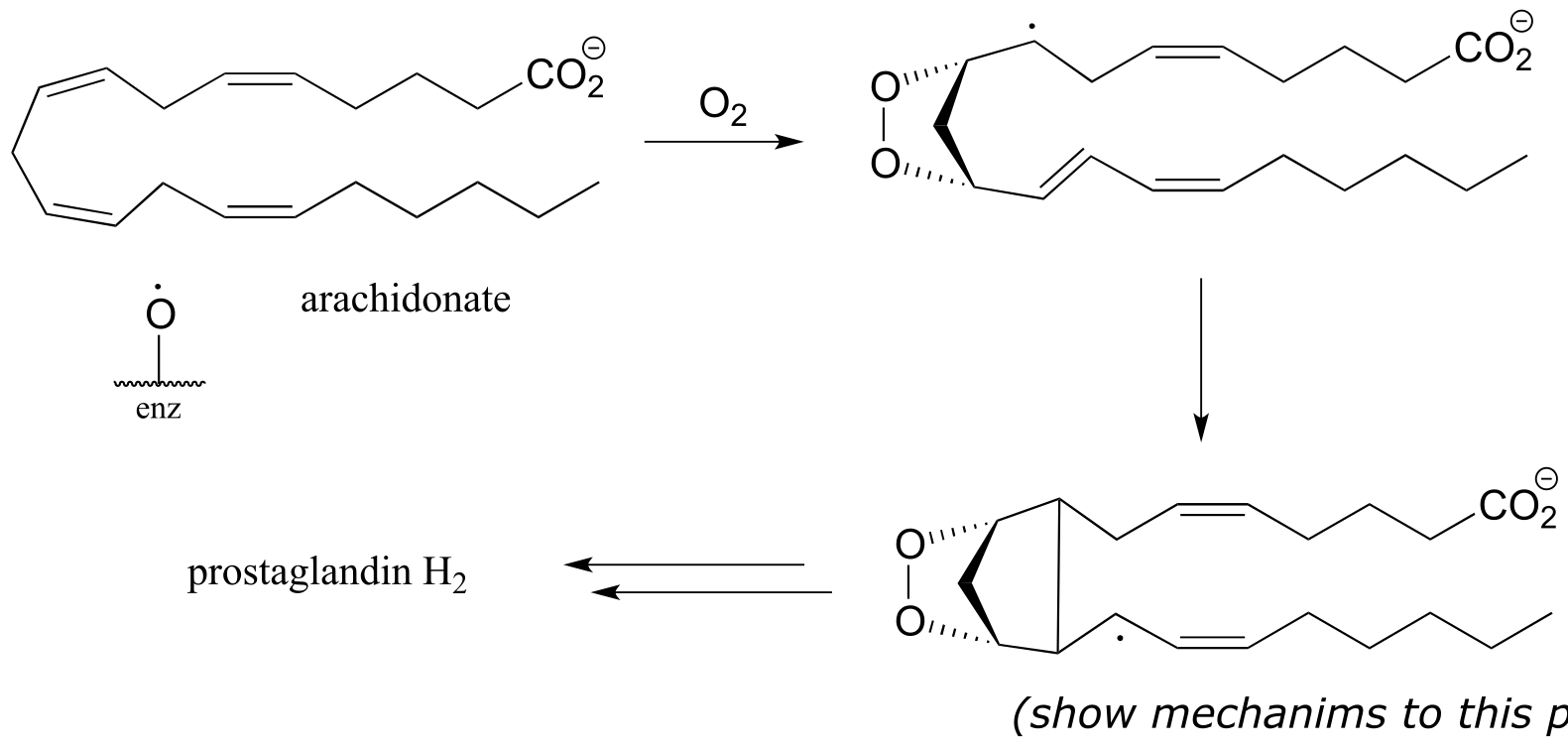
Propose a mechanism for the steps of the reaction that are shown in this figure.
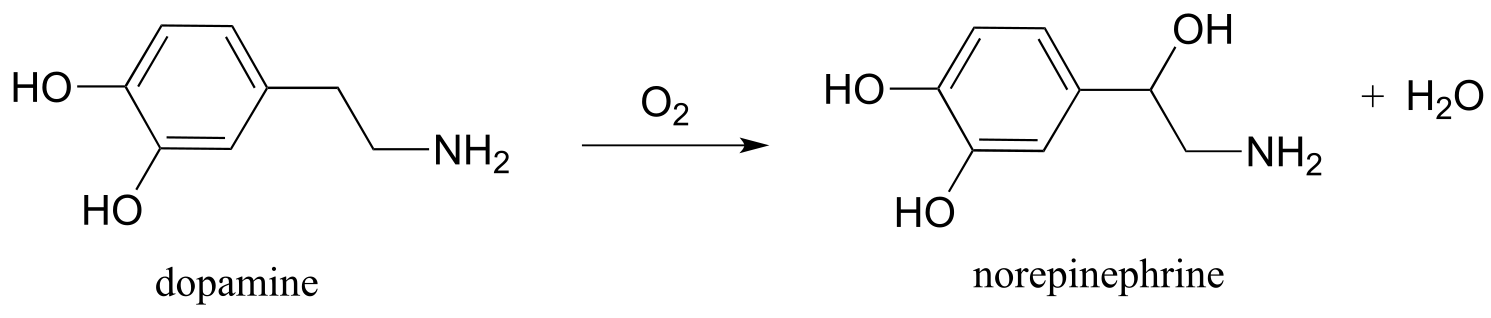
P16.9: Some redox enzymes use copper to assist in electron transfer steps. One important example is dopamine β-monooxygenase (EC 1.14.1.1), which catalyzes the following reaction:
The following intermediates have been proposed: (see Biochemistry 1994, 33, 226) Silverman p. 222
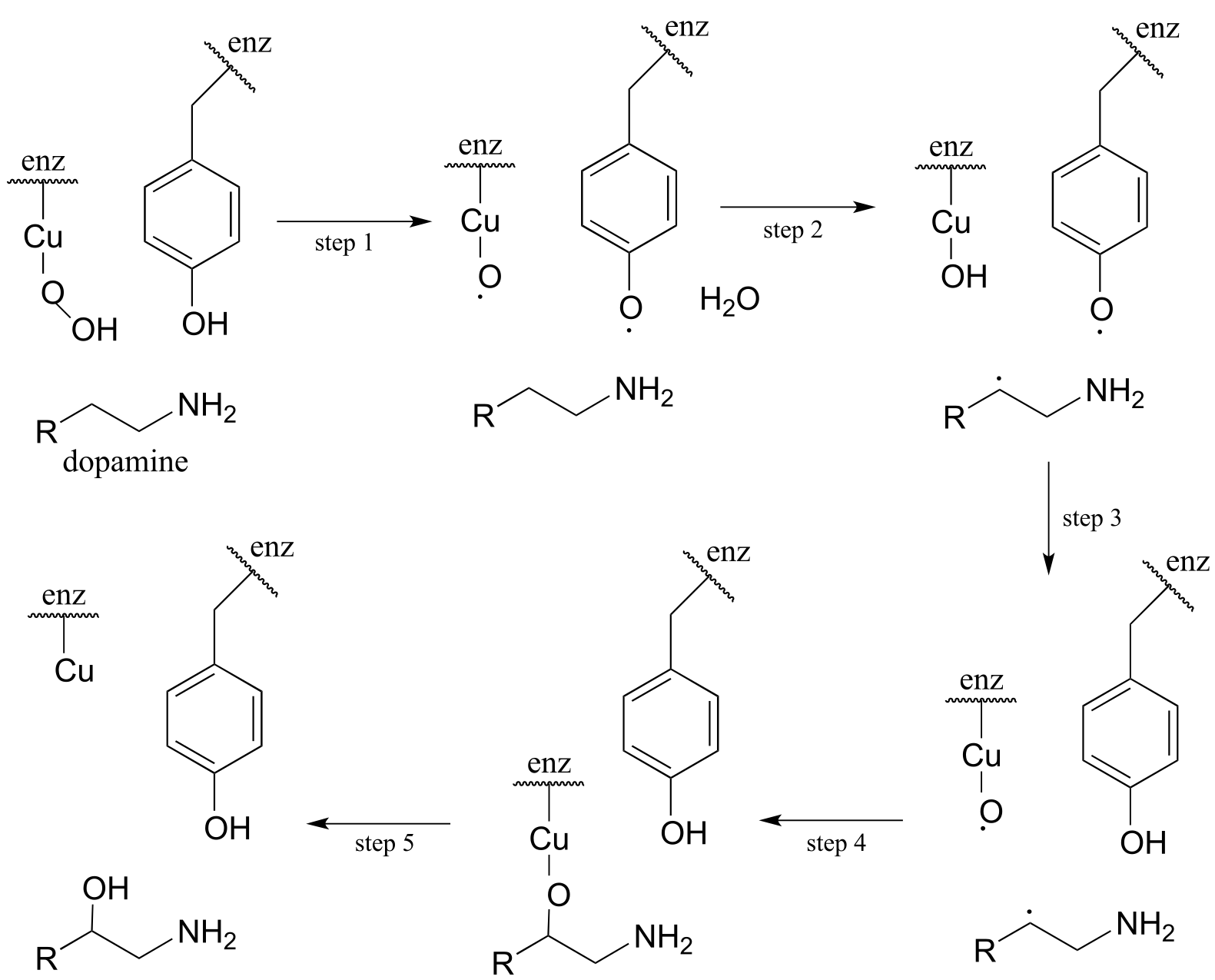
Draw mechanistic arrows for steps 1-4.